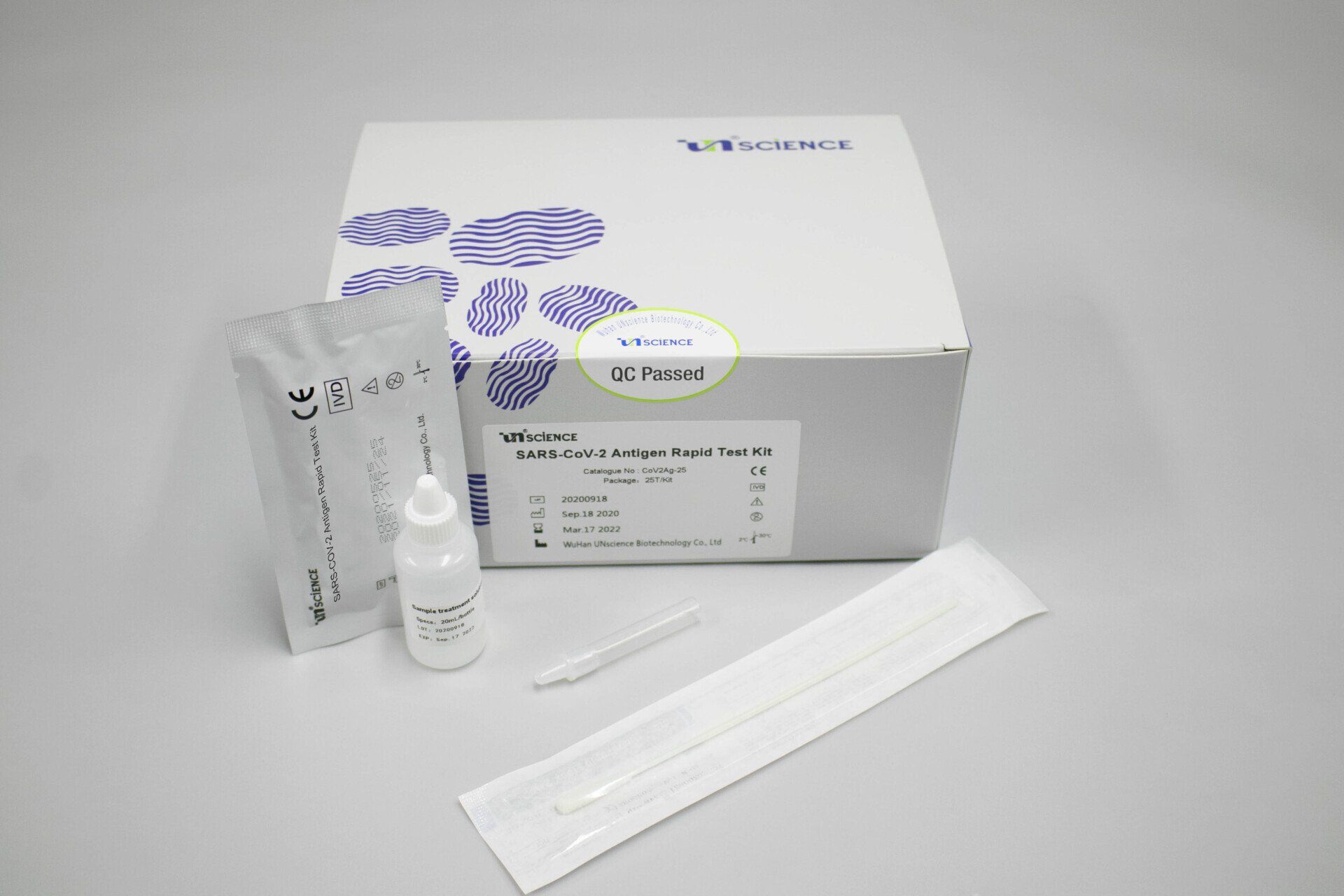
Covid-19 Antigen
Rapid Test Kit - CE IVD
This product is used for in vitro qualitative detection of novel coronavirus (SARS-CoV-2) antigen in human oropharyngeal swabs, nasal swabs and nasopharyngeal swabs.
This product is used under medical institutions only.
INTENDED USE
This product is used for in vitro qualitative detection of novel coronavirus (SARS-CoV-2) antigen in human oropharyngeal swabs, nasal swabs and nasopharyngeal swabs. This product is used under medical institutions only.
The SARS-CoV-2 is a new type of coronavirus and named by the World Health Organization. The SARS-CoV-2 has spread all over the world. It causes viral pneumonia with fever, fatigue, dry cough and sore throat as the main manifestations. The severe cases of viral pneumonia caused by it manifested as dyspnea, decreased blood oxygen saturation, and rapid development of acute respiratory distress syndrome, septic shock, etc. In serious cases, metabolic acidosis and coagulation dysfunction are difficult to be treated, which directly affect life and health.
Covid Antigen Rapid Test Kit
TEST PRINCIPLE
This kit adopts the sandwich method and the technical principle of colloidal gold immunochromatography to qualitative determine the SARS-CoV-2 antigen.
During the test, the sample is dropped into the sample well, and chromatography is performed under the capillary effect. The SARS-CoV-2 antigen in the sample combined with the colloidal gold-labeled SARS-CoV2 monoclonal antibody I, and then spread to the test area.
It is captured by another coated antibody (SARS-CoV-2 monoclonal antibody II), to form a complex and gather in the test area (T line).
The quality control area is coated with the goat anti-mouse antibody, and the colloidal gold-labeled antibody is captured to form a complex and aggregate in the quality control area (C line). If the C line does not show color, it indicates that the result is invalid, and this sample needs to be tested again.
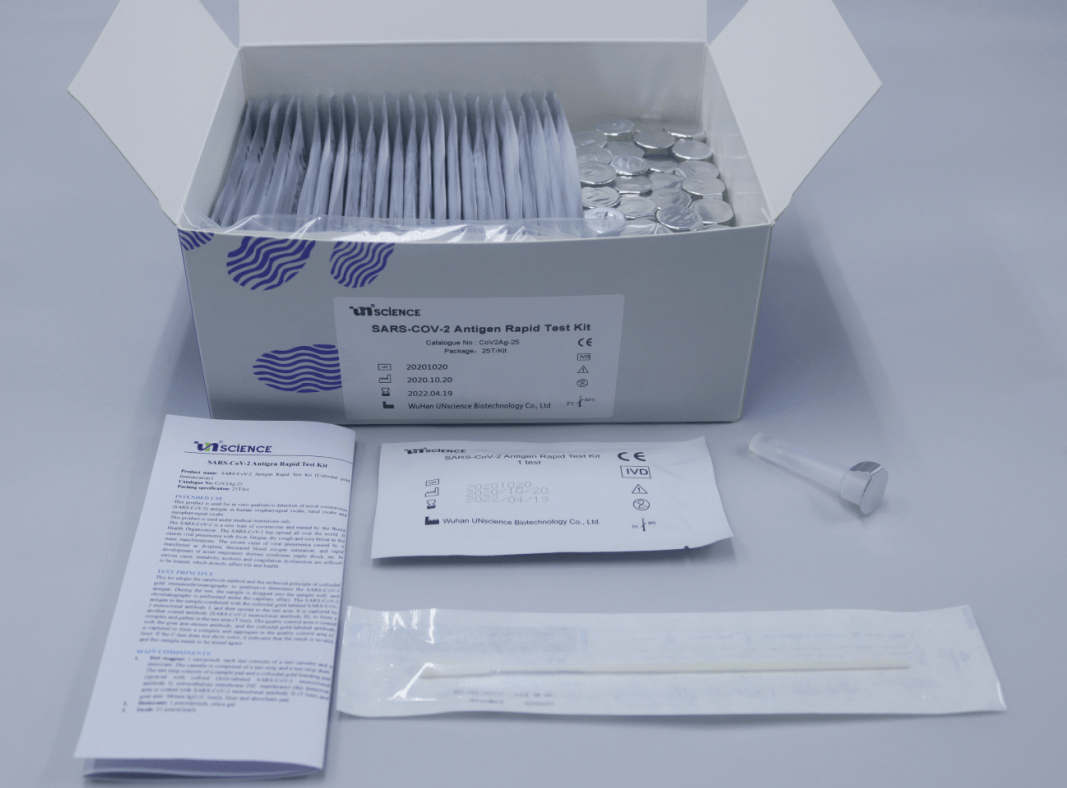
Cod. CoV2Ag-25
Product name: SARS-CoV-2 Antigen Rapid Test Kit (Colloidal gold Immunoassay)
Packing specification: 25T/kit
Format: Cassette
Reading Time: 10 minutes
Lead Time:
In stock
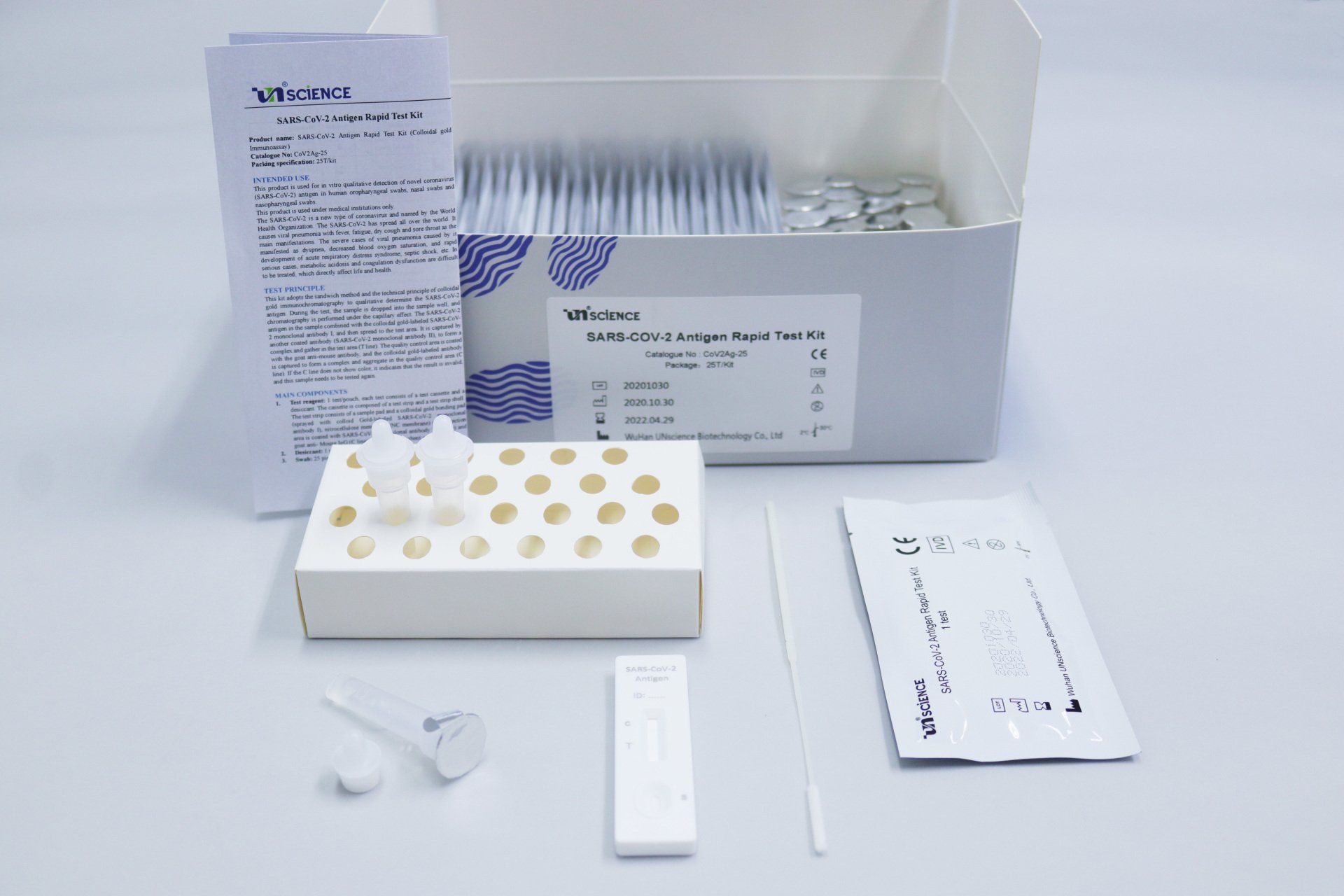
Main components
1. Test reagent: 1 test/pouch, each test consists of a test cassette and a desiccant. The cassette is composed of a test strip and a test strip shell. The test strip consists of a sample pad and a colloidal gold bonding pad (sprayed with colloid Gold-labeled SARS-CoV-2 monoclonal antibody I), nitrocellulose membrane (NC membrane) (the detection area is coated with SARS-CoV-2 monoclonal antibody II (T line) and goat anti- Mouse IgG (C line)), liner and absorbent pad.
2. Desiccant: 1 piece/pouch, silica gel
3. Swab: 25 Pieces/pack
4. Sample treatment solution: 20 mL/bottle
5. Sampling tube: 25 pieces/pack.
Storage and stability
Storage:
The test reagent is stored at 2℃-30℃, and the validity period is tentatively set for 18 months. See the label for the production date and expiration date.
Sample Requirements
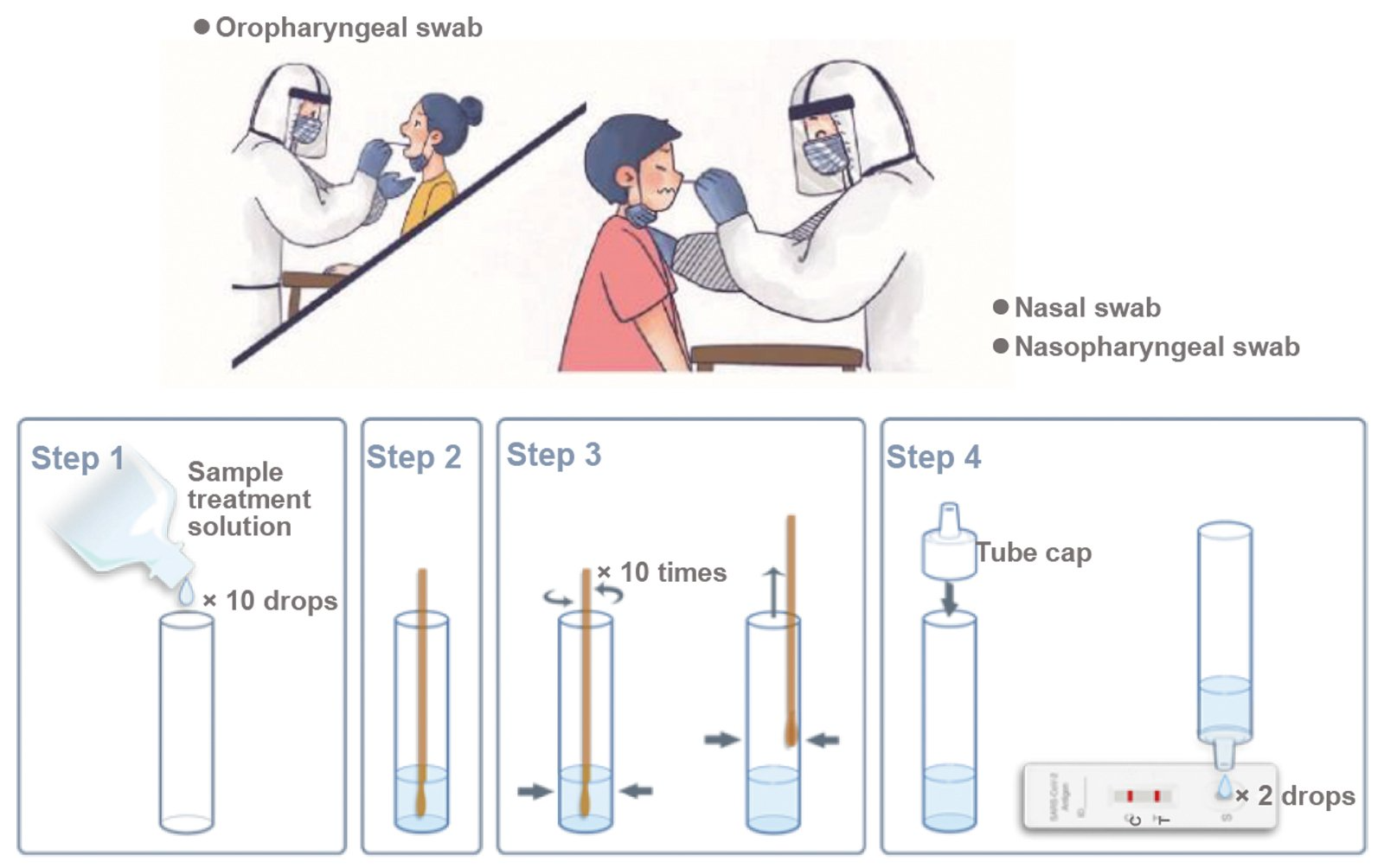
1. Oropharyngeal swab:
The head of the person is slightly tilted, with mouth wide open, exposing the pharyngeal tonsils on both sides. Use the swab to gently wipe the tonsils on both sides for at least 3 times, and then wipe the posterior pharyngeal wall up and down at least 3 times.
2. Nasal swab:
Prior to collecting the nasal swab, the patient should be instructed to blow their nose. Carefully insert the swab into the nostril with the most secretion under visual inspection. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril), and rotate the swab against the nasal wall several times and then remove it from the nostril.
3. Nasopharyngeal swab:
Carefully insert the swab into the nostril with the most secretion under visual inspection. Keep the swab near the septum floor of the nose while gently pushing the swab into the posterior nasopharynx. Rotate the swab several times then remove it from the nasopharynx (in case of reflex cough, stop for 1 minute).
Sample preparation
- Take out sampling tube and add 10 drops of sample treatment solution.
- Put the swab into sampling tube, make sure the swab soaked in the solution.
- Rotate and squeeze the swab on the wall and bottom of the tube 10 times, squeeze the swab tip along the inner wall of the sample tube to keep as much solution in the tube as possible.
- Remove the swab. It is recommended to test immediately after sample collection and processing. If the test cannot be performed timely, the processed samples can be stored at 2-8℃ for 48h.
Test Procedure
Before use, please read the instructions carefully and operate in strict accordance with the instructions:
- Bring the pouch to room temperature before use.
- Take out the cassette, put it on a horizontal table.
- Add 2 drops of the processed sample vertically into the sample well and start the timer.
- Observe the result after 10 minutes, the result is valid within 30 minutes, read results after 30 minutes is invalid.
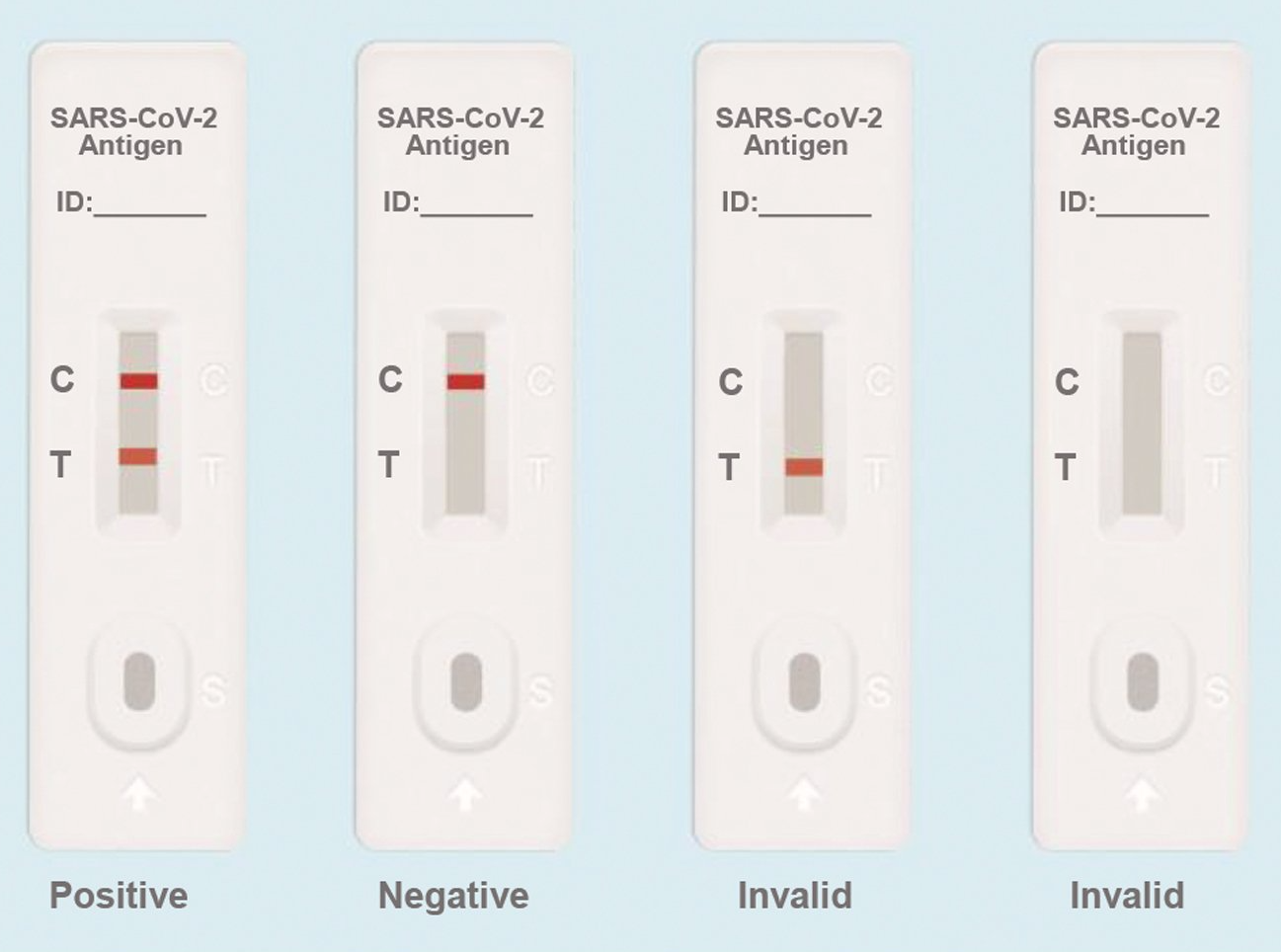
Interpretations of Result
- Positive: Both the detection line (T line) and the quality control line (C line) appear colors.
- Negative: The test line (T line) does not appear color, only the quality control line (C line) appears color.
- Invalid: The quality control line (C line) does not appear color, which means that the test is invalid and the test should be repeated.
NOTE: This figure is only used as a reference.
Product performances, limitations and notes:
Clinical Report:
EC Certificate:
FAQ
- For what purpose actually these tests (Cov2Ag-25) are used?
SARS-CoV-2 Antigen Rapid Test Kit (Colloidal gold Immunoassay) is a rapid chromatographic immunoassay for the qualitative detection of the novel coronavirus (SARS-CoV-2) antigen in human oropharyngeal swab, nasal swab and nasopharyngeal swab in vitro. It cannot be used as a basis for the diagnosis and exclusion of novel coronavirus pneumonia and is not suitable for screening of the general population.
- How soon after infection will the test work?
SARS-CoV-2 Antigen Rapid Test Kit (Colloidal gold Immunoassay) are not suitable for new virus/positive patients (no positive results) and are generally used at the onset of infection and symptoms (1-7 days).
- Product Advantage
- No special equipment needed.
- Easy to use.
- Results in 10 minutes.
- Intuitive visual interpretation.
- Results were validated by PCR and Clinical diagnosis.
- Works with oropharyngeal swab, nasal swab and nasopharyngeal swab sample types.
- Which Antigen/Antibody is applied in the Cov2AG-25?
The colloidal gold labeled reagent coated on conjugate pad is SARS-CoV-2 monoclonal antibody I.
T Line: SARS-CoV-2 monoclonal antibody II.
C Line: Goat anti-mouse IgG (polyclonal).
- What kind of swab is routinely supplied with the kit?
Oropharyngeal swab.
Limitations of SARS-CoV-2 Antigen Rapid Test Kit
- This kit is a qualitative test for in vitro auxiliary diagnosis.
- Due to methodological limitations, the sensitivity of this kit is lower than that of PCR. Therefore, more attention should be paid to the negative results of this experiment, and a comprehensive judgment should be combined with other test results. It is recommended that the suspected results be supplemented with nucleic acid testing or virus isolation and culture in vitro for confirmation.
- Unreasonable sampling, transportation and handling, or low virus content in the sample will lead to false negative results.
- The test results of this reagent are for clinical reference only and cannot be used as the only basis for clinical diagnosis. The tester should conduct a comprehensive evaluation based on the patient's clinical manifestations and other laboratory test results.
RICHIEDI OFFERTA
Ti risponderemo il più presto possibile.
Riprova in un secondo momento.
Contatti
Sede Operativa:
IIIª TRAVERSA PISCIARELLI 18/A, 80078 – POZZUOLI (NA)
Numero Verde
Orari: 08.45 - 13,30 / 14.30 - 17.45
Email:
Info: microtech@microtech.eu
Preventivi:
offerte@microtech.eu
Ordini:
commerciale@microtech.eu
Amministrazione:
amministrazione@microtech.eu
Magazzino: magazzino@microtech.eu
Informazioni
Sede Legale:
VIALE AUGUSTO 162, 80125 - NAPOLI (NA)
P. IVA 05791560633
Cap. Soc. Int. Versato € 100.000
Nr. REA NA-459230-S.R.L.
Cod. SDI M5UXCR1
Cod. NSO CAW8PERJ
Id. Peppol 0210:05791560633